Stem Cell Hair Loss Treatments: What You Need to Know
The word “stem cells” makes any product or treatment appear advanced, as if it came from the cutting edge of research. Many people are desperate to try any treatment to help them with their hair loss because there are so many goods and services that use the term “stem cell hair restoration”.
As a practicing surgeon for over 25 years and the founder of TrichoStem Hair Regeneration, where we have been helping people with male and female pattern hair loss since 2011 with a wound healing technology that recruits your adult stem cells from your body, I feel compelled to provide some perspective and context in what appears to be a free-for-all in stem cell marketing.
What is Stem Cell?
According to the National Institutes of Health: “Stem cells are a specific type of cell capable of evolving into many different types of specialized cells within the body. There are three primary types of stem cells: embryonic stem cells are characterized as pluripotent in nature—capable of developing into the two hundred or so specialized cells of the adult organism; adult stem cells exist within certain tissues of the body (for example, blood and bone marrow) and carry out repair and regenerative functions; and induced pluripotent stem cells (iPSCs) are adult stem cells that have been genetically reprogrammed to behave like embryonic stem cells.”
Anyone without a scientific background may find this definition confusing. In order to protect the public, the FDA has issued declarations about stem cell therapy. They specifically issued a statement titled “Consumer Alert on Regenerative Medicine Products Including Stem Cells and Exosomes” dated July 22, 2020.
In this statement, they declared the following: Stem cell products are regulated by FDA, and generally, all stem cell products require FDA approval. Currently, the only stem cell products that are FDA-approved for use in the United States consist of blood-forming stem cells (also known as hematopoietic progenitor cells) that are derived from umbilical cord blood. These products are approved for use in patients with disorders that affect the production of blood (i.e., the “hematopoietic” system) but they are not approved for other uses. The FDA also regulates exosome products. In general, exosome products designed to treat human diseases or diseases must be approved by the FDA. There are presently no FDA-approved exosome products.
The Role of Exosomes and Liposomes Technology in Cell Therapy
Exosomes, and liposomes are technologies that enable more direct delivery of certain medications or other materials into cells for therapeutic use. I believe that this technology has a lot of potential. As far as I’m concerned, the therapeutic effects of the precise materials put into your cells via these technologies must be investigated for safety and efficacy, just like any pharmaceutical or biologic agent.
There has been some interest in adipose-derived stem cells. Procedures such as “stem cell facelifts” or “stem cell hair loss treatments” are based on a process in which fat is extracted through a surgery similar to liposuction, then processed for reintroduction for stem cell-induced advantages. In terms of adipose-derived stem cells for hair loss, limited trials have found little efficacy, and this type of surgery is no longer as “cutting edge” as it once was.
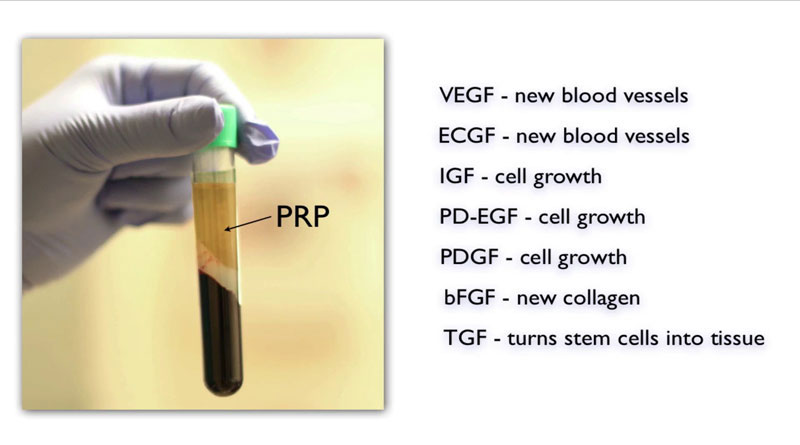
PRP and Stem Cells for Healing
When you sustain an injury such as a cut, platelets in your blood are triggered to seal the wounded vessel and recruit your body’s adult stem cells for wound repair.
Since the late 1990s, dentistry, orthopedic surgery, and, more recently, aesthetic medicine have used platelet-rich plasma to control the recruitment of adult stem cells from your own body for benefits tailored to the patient’s individual needs.
PRP Alone for Hair Loss Treatment
PRP alone administered into the scalp has been shown in published research to stimulate short-term hair growth in persons suffering from genetic pattern hair loss. Unfortunately, using PRP alone to treat hair loss is restricted because it requires regular scalp injections every 1-3 months. We encounter a lot of people who are frustrated with the limited results and pain associated with regular PRP treatments.
I must admit that the “discovery” of PRP for hair loss is somewhat ironic, as we had been using PRP as part of our TrichoStem Hair Regeneration System for many years before my colleagues decided to embrace this “new” technology. Many of these doctors transitioned from PRP doubters to PRP promoters.
PRP+ACell for Hair Loss Treatment
Since 2011, we have been performing TrichoStem Hair Regeneration treatments using our unique ACell+PRP protocols tailored to your specific hair loss profile. Acellular matrix is commonly referred to as ACell, which is the company that manufactures this wound healing technology developed from pig bladder. The FDA has cleared Acell’s Micromatrix product for use in wound healing applications. The wound healing benefits appear to be attributable to an increase in your body’s adult stem cell response to an injury. This was the foundation for our early use of Micromatrix during hair transplant surgery.
A year after hair transplant surgery, areas that were not transplanted showed substantial regrowth and thickening of the patient’s natural hair.
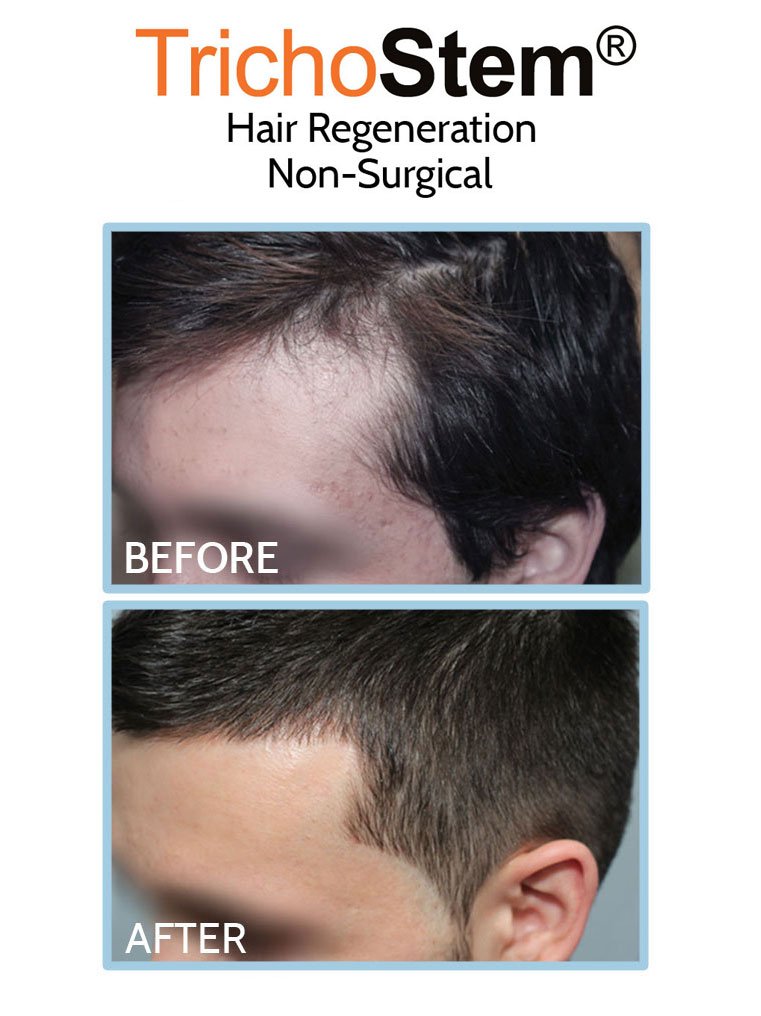
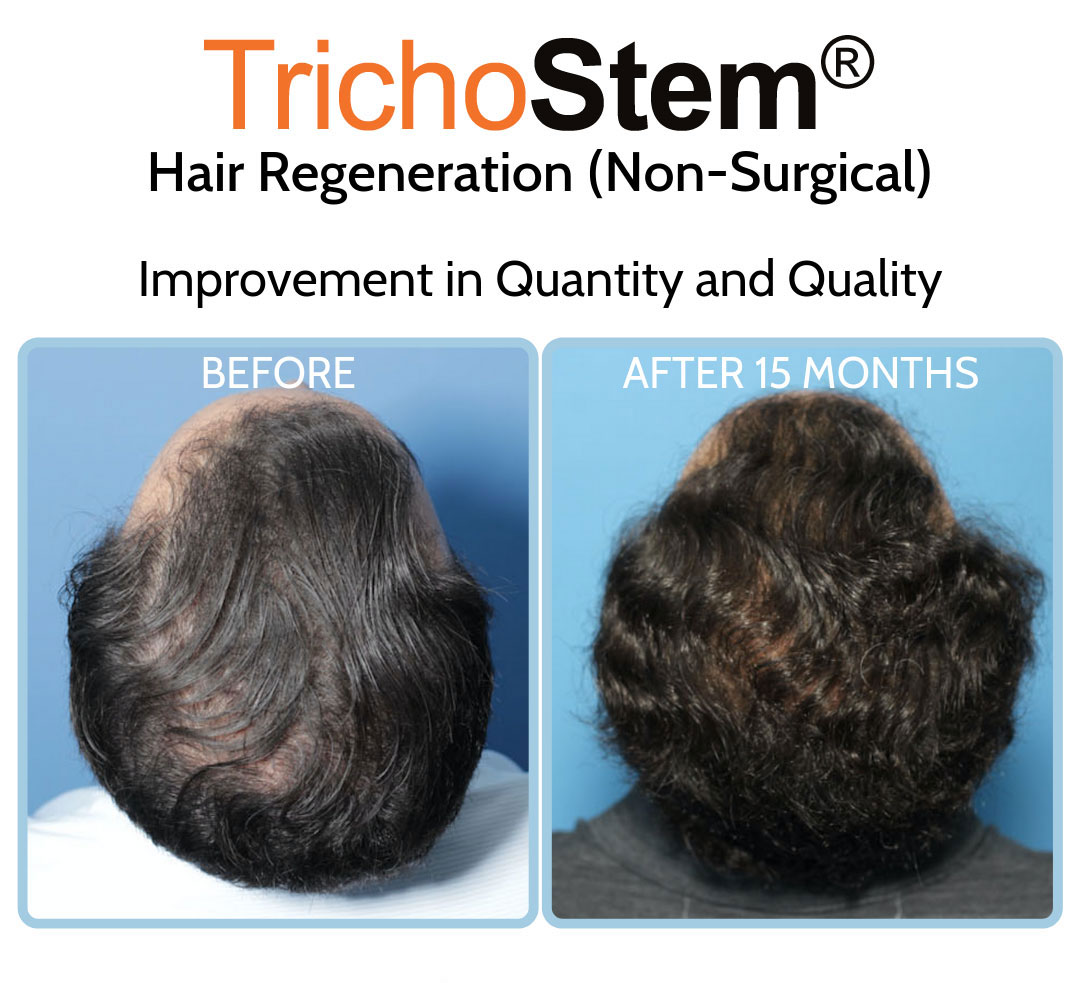
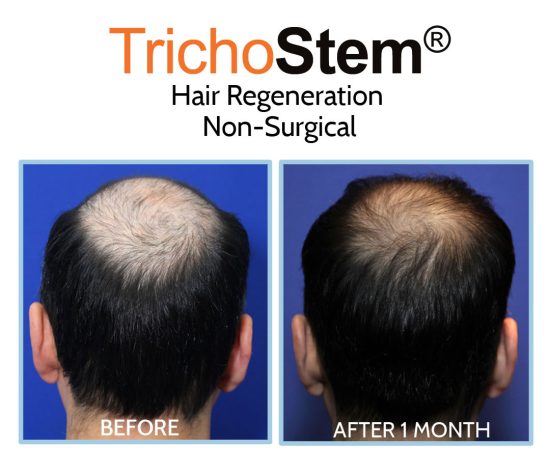
This led me to create a system of personalized formulations and delivery systems combining Acellular matrix and PRP for the non-surgical treatment of male and female pattern hair loss, as well as other hair loss disorders such as traction alopecia. A normal TrichoStem Hair Regeneration treatment schedule includes only 1-2 treatment sessions, with the second treatment taking place 15-24 months following the first. The treatment plan’s benefits often last 3-5 years or longer, with over 99% of patients experiencing an improvement in scalp coverage.
Finally, a word of caution: there are several “stem cell” hair loss remedies for sale online. These products generally fail to meet any of the stem cell standards previously established by the FDA and the NIH. Many of these goods will be labeled with the disclaimer, “This statement has not been evaluated by the FDA.” “This product is not intended to diagnose, treat, cure, or prevent disease.” This disclaimer is common for reputable products like vitamin supplements so it is not always a warning indicator. However, when it comes to products claiming to have stem cells, which can be placed in virtual shopping carts, caveat emptor or buyer beware.
I hope this information from my experience was helpful to you. Thank you.
Hair Loss Specialists Manhattan NYC and Long Island, New York
Dr Amiya Prasad has practiced hair restoration, including surgical hair transplants, in New York City and Long Island for over 25 years, and is the founder of TrichoStem Hair Regeneration – a non-surgical hair loss treatment for men and women.
If you’d like to receive recommendations for your specific situation, please fill out the form below, or call our offices in Manhattan, New York City at (212) 265-8877; or Garden City, Long Island at (516) 742-4636; or Vienna, Virginia at (703) 356-1336 to book a consultation.

